Protect Yourself from Tooth Decay: Remineralization Strategies
Nanohydroxyapatite and Calcium Phosphate Ions
Dr. Philip Marsh first reported the biofilm nature of dental caries and prolonged periods of low pH, not sugar availability, as being responsible for the increase in acidic bacteria in the biofilm.1 The shift in pH alters the components in the biofilm and leads to demineralization. In a healthy individual, following acid challenges, buffering agents and bacteria in the saliva and biofilm raise the pH and return it to a healthy pH, and then remineralization occurs.1 This cycle repeats continuously throughout the day in our mouths. In a healthy mouth, there is a balance between episodes of demineralization and remineralization with no resulting mineral loss from the teeth. This normal pH cycling in the mouth is considered healthy, where mineral is lost during periods of low pH and returns to the teeth during periods of higher pH. In dental caries, there is a loss of this balance with prolonged periods of low pH, favoring demineralization.
The body maintains healthy tooth mineral by replacing the lost hydroxyapatite and fluorapatite crystal particles as soon as the pH rises above critical remineralization pH.2
Remineralization occurs as a natural process within a healthy individual’s mouth, and for this reason, some products are designed to mimic this process for the purpose of prevention and therapeutic strategies for dental caries. Remineralization is a complex process involving the condition of the existing tooth structure, the quantity and quality of saliva, the content and behavior of the bacteria biofilm, the presence of fluoride, and oral pH. In order to understand remineralization, it is important to understand how tooth structure is designed and how the natural process works. Structurally, enamel is basically a crystal. When teeth form, they go through a process called amelogenesis, which is a process of crystal growth. In nature, crystals grow when in the right environment; nanoparticles of the crystal that are in a liquid or gas around a larger crystal move toward larger bodies of crystal and attach themselves. This is described as “oriented attachment”; the nano-sized crystals self-orient and attach themselves to larger crystals. Remineralization of the teeth works in the same way. The body maintains healthy tooth mineral by replacing the lost hydroxyapatite and fluorapatite crystal particles as soon as the pH rises above the critical remineralization pH.2 For hydroxyapatite, this pH is 5.5, while for fluorapatite, the critical pH is 4.5. The basic building block of enamel is a crystallite about 20nm in size.
Ionic Forms of Calcium Phosphate
Saliva is the solution around the tooth that allows for remineralization as it is supersaturated with brushite, octacalcium phosphate, tricalciumphosphate, fluorapatite, and hydroxyapatite nanoparticles—all forms ofcalcium phosphate.3 Understanding this, and in an attempt to mimic natural remineralization, various forms of calcium phosphate ions have been included in some therapeutic strategies in addition to fluoride. Tricalcium phosphate, amorphous calcium phosphate, and CPP/ACP (casein phosphopeptide coated amorphous calcium phosphate) have all been added to different oral care products.4, 5, 6 The scientific results in these topics are mixed; some conclude there is a lack of evidence or no benefit, while others demonstrate an improved result.7, 8, 9 , 10 While some have thought that calcium and phosphate are present in ionic forms in saliva, some of the mixed study results may be due to the fact that ionic forms of calcium phosphate generally do not occur in saliva unless salivary pH is influenced by outside sources, as the pH of resting saliva is about 6.75 and the pH of stimulated saliva is about 7.8, whereby the forms of calcium phosphate would only be available in nanocrystallite particle form. The biomimetic form of calcium phosphate is a 20 nm particle of hydroxyapatite.
 Nanoparticles of Hydroxyapatite
Nanoparticles of Hydroxyapatite
Other non-ion forms of calcium phosphate materials have been used and studied as well. As hydroxyapatite and fluorapatite are present in greatest quantities in saliva and are the most bioavailable form for remineralization, they play the most significant role in remineralization. Hydroxyapatite and fluorapatite are present in supersaturated levels in saliva, and as the saliva flow increases during stimulated saliva flow, the degree of supersaturation also increases. Hydroxyapatite in nanoparticle crystallite form is also the most thermodynamically stable form of calcium phosphate11, and studies examining nanoparticle hydroxyapatite as a biomimetic remineralization agent have been performed recently.12,13,14 Studies demonstrate that nanoparticles in the 20 nm size (1/850th the width of a human hair) mimic the building blocks of natural enamel and are effective as an enamel repair material and anticaries agent.15 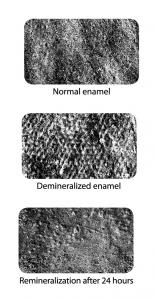 Nanoparticle hydroxyapatite has also been demonstrated to restore luster to enamel damaged by bleaching agents.13
Nanoparticle hydroxyapatite has also been demonstrated to restore luster to enamel damaged by bleaching agents.13
Numerous choices exist for patients looking to combat their risk factors with protective factors and weight their personal “caries balance” toward health. When considering a caries management therapy, always consult your dental professional and choose the most proactive therapy they recommend. On the right in Figure 16 (scanning electron microscope) are images of extracted teeth used in a study on the remineralization potential of a nanohydroxyapatite gel used in conjunction with 1.1% neutral sodium fluoride, pH neutralization (pH of 9), and xylitol (such as CTx4 Gel 1100). The first image shows the natural tooth surface, the second shows artificial demineralization using 35% hydrogen peroxide, and the third shows the remineralization and attached particles of nanohydroxyapatite after the demineralized surface was brushed with the gel and placed in artificial saliva for 24 hours. Clinical trials are a challenge in examining remineralization, and it is difficult to duplicate in vivo (human) conditions during an in vitro (laboratory) study. Remineralization research is often limited to models with artificial lesions and saliva (as shown).16, 17
- P. D. Marsh, “Dental Plaque as a Biofilm and a Microbial Community—Implications for Health and Disease,” BMC Oral Health 6, Suppl. 1(2006): S14.
- A. L. Boskey and A. S. Posner, “Conversion of Amorphous Calcium Phosphate to Microcrystalline Hydroxyapatite: A pH Dependent, Solution Mediated, Solid-Solid Conversion,” Journal of Physical Chemistry 77, no. 19 (1973): 2313.
- R. Štulajterová and L. Medveckýa, “Effect of Calcium Ions on Transformation Brushite to Hydroxyapatite in Aqueous Solutions,”Colloids and Surfaces A: Physicochemical and Engineering Aspects 316, no. 1 (2008): 104–109.
- R. L. Karlinsey, A. C. Mackey, E. R. Walker, and K. E. Frederick, “Surfactant-Modified ß-TCP: Structure, Properties, and In Vitro Remineralization of Subsurface Enamel Lesions,” J Mater Sci 21(2010): 2009–2020.
- R. L. Karlinsey, A. C. Mackey, G. K. Stookey, and A. M. Pfarrer, In Vitro Assessments of Experimental NaF Dentifrices Containing a Prospective Calcium Phosphate Technology,” Am J Dent 22 (2009):180–184.
- E. C. Reynolds, “Casein Phosphopeptide-Amorphous Calcium Phosphate: The Scientific Evidence,” Adv Dent Res 21 (2009): 25–29.
- A. Azarpazhooh and H. Limeback, “Clinical efficacy of Casein Derivatives: A Systematic Review of the Literature,” J Am Dent Assoc 139 (2008): 915–924.
- M. V. Morgan, G. G. Adams, D. L. Bailey, C. E. Tsao, S. L. Fischman,and E. C. Reynolds, “The Anticariogenic Effect of Sugar-Free Gum Containing CPP-ACP Nanocomplexes on Approximal Caries Determined Using Digital Bitewing Radiography,” Caries Res 42(2008): 171–184.
- S. Lata, N. O. Varghese, and Jolly Mary Varughese, “Remineralization potential of fluoride and Amorphous Calcium Phosphate-Casein Phospho Peptide on Enamel Lesions: An In Vitro Comparative Evaluation,” J Conserv Dent 13, no. 1 (2010): 42–46.
- L. J. Walsh, “Evidence That Demands a Verdict: Latest Developments in Remineralization Therapies,” Australasian Dental Practice (March/April2009): 48–59.
- T. Tanaka, N. Yagi, T. Ohta, Y. Matsuo, H. Terada, K. Kamasaka, K. To-o, T. Komentani, and T. Kuriki, “Evaluation of the Distribution and Orientation of Remineralized Enamel Crystallites in Subsurface Lesions by X-ray Diffraction,” Caries Res 44, no. 3 (2010): 253–9.
- R. Takikawa, K. Fujitsu, T. Ishizaki, and R. E. Hayman, “Restoration of Post-bleach Enamel Gloss Using a Non-abrasive, Nano-hydroxyapatite Conditioner,” J Dent Res Special Issue B (Brisibane Abstracts; 2006): 85.
- S. B. Huang, S. S. Gao, and H. Y. Yu, “Effect of Nano-hydroxyapatite Concentration on Remineralization of Initial Enamel Lesion In Vitro,”Biomed Mater 4, no. 3 (2009): 034104.
- L. Li, H. Pan, J. Tao, X. Xu, C. Mao, X. Gu, and R. Tang, “Repair of Enamel by Using Hydroxyapatite Nanoparticles as the Building Blocks,” J of Mater Chem 18 (2008): 4079–4084.
- R. P. Allaker, “The Use of Nanoparticles to Control Oral Biofilm Formation,” J Dent Res 89, no. 11 (2010): 1175–1186.
- R. Asa, “Proactive Prevention: Treating Caries Disease with Remineralization,” AGD Impact Journal 39, no. 2 (February 2011):20–24.
- N. Roveri, E. Battistella, C. L. Bianchi, et al., “Surface Enamel Remineralization: Biomimetic Apatite Nanocrystals and Fluoride Ions Different Effects,” J of Nanomaterials (2009): 1–9.
